Abstract
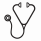
Background
Eltrombopag (ELT), an oral thrombopoetin-receptor agonist, has recently emerged as an encouraging and promising agent in acquired aplastic anemia. It has shown efficacy within clinical trials exploring its role in the first - line therapy in addition to standard immunosuppressive therapy as well as in the refractory setting (Townsley et al, NEJM 2017; Olnes et al, NEJM 2012). How ELT is used outside of clinical trials in the real-world setting after approval in Europe and results of this treatment are not known.
Methods
We conducted a retrospective survey on the use of thrombopoietin agonists for AA among EBMT member centers. Thirty-one centers provided data on 137 patients treated between 11/2011 to 10/2017. We merged our dataset with the cohort from the French Reference Center for Aplastic Anemia (Lengline et al., Haematologica 2017). Here we report the outcome 165 patients having received at least 30 days ELT and having a follow up of at least 2 months, patients with a shorter duration of treatment or follow up, as well as three patients receiving romiplostim were excluded. The following response criteria were used: complete response (CR) defined as hemoglobin >100g/l, neutrophil count >1.5 x106/ml and platelet count >100 x106/ml; partial response (PR) corresponding to transfusion independence and minimal response (MR) to some improvement in one or more lineage but not fulfilling the criteria of PR.
Results
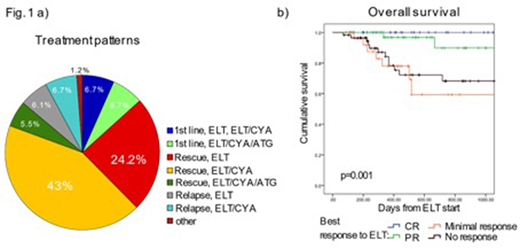
The median follow-up was 14 months (IQR 7-24 months). Before starting ELT, AA was classified as severe or very severe in 68.6% respectively 13.8 % of patients. At last follow-up 87.3% of patients were alive, of those 47.2% continued ELT (maximum median dose: 150mg/day). A minority of the patients (n=22, 13.3 %) received ELT upfront, i.e. within 60 days following initiation of first-line treatment, whereas most patients were treated with ELT as a rescue treatment either for insufficient response to previous treatment(s) (n= 122, 73.9%) or relapse (n=21, 12.7%). ELT was applied both as monotherapy (n= 56, 33.9%) and in combination with IS (ELT+ CYA: n= 87/52.7%, ELT+CYA/ATG: n=20/12.1% , Fig. 1 a). Importantly, 69.7% of patients received ELT outside the FDA/EMA label, either in the first line setting or as part of a combination therapy.
The reported overall response rate in our cohort was 64.6 %, with 19.5 % CR, 28% PR and 17.1% minimal response. Best response was achieved in median after 252 days (95% CI 181-366 days).
In univariate models, we could not identify any baseline characteristics associated with better response in the whole cohort, although severity of AA before start of ELT was significantly associated with death without a response (p= 0.024). In the multivariate model, combination therapy was a predictor (p=0.049) of response, with a hazard ratio for response of 3.32 for the ELT/CYA/ATG group compared to ELT monotherapy (p=0.008). In the subgroup of patients with previous therapy, response to previous therapy was a strong predictor of response (HR 2.2, p<0.001), whereas previous exposure to ATG, interval from diagnosis or interval from last treatment were not significantly associated with response.
The median survival was not reached in our cohort, the 1-year survival from start of ELT was 87.9%. Twenty-one (12.7 %) patients died during follow-up, seven (33%) of these had undergone allo-HSCT and in six (28.6%) deaths were classified as HSCT-related.
In univariate and multivariate analysis, AA severity at ELT start and response to ELT were associated with OS. Interestingly, the better survival of patients responding to ELT was driven by CR and PR, as patients with a minimal response to ELT had the same survival as patients without a response (Fig 1b.).
Adverse events (AEs) were reported in 30.6% of patients, although severe (grade III-IV) AEs were rare (8.9%) and ELT was stopped only in 2 patients due to AEs.
Cytogenetic abnormalities were assessed systematically only at diagnosis and showed a normal karyotype in 70.9% of patients and an abnormal karyotype in 11 cases (7.9%) including 3 cases of trisomy 8 and 1 case of inv(3;3q26). Only 2 cases of MDS diagnosed 2 and 5.5 months after ELT start were reported.
Conclusions
ELT is used widely in Europe to treat AA patients, mostly in the relapsed/refractory setting. Whereas patients achieving at least transfusion independence with ELT have an excellent survival, the outcome of patients refractory to ELT remain unsatisfactory.
Cortelezzi:janssen: Consultancy; abbvie: Consultancy; novartis: Consultancy; roche: Consultancy. Gaidano:Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Morphosys: Honoraria; Gilead: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Peffault De Latour:Pfizer Inc.: Consultancy, Honoraria, Research Funding; Alexion Pharmaceuticals, Inc.: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract